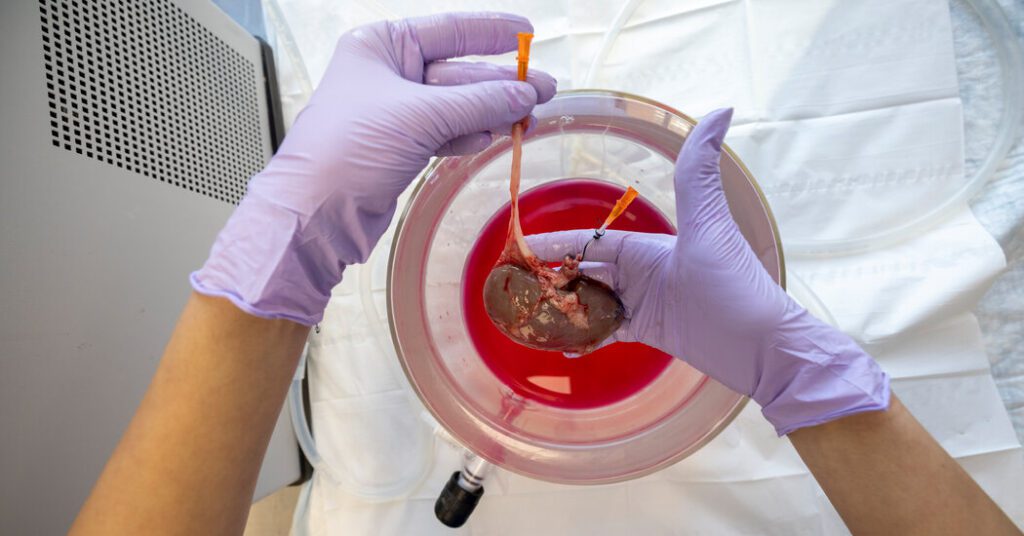
On the last day of March, surgeons at Massachusetts General Hospital began surgery that they hoped would lead to lasting changes in the way the kidneys are implanted in people.
The patient that morning was not a human. It was a pig and was anesthetized on the table. The pig had one kidney missing and needed an implant.
Kidneys usually need to be transplanted within 24-36 hours, but the kidneys that enter the pig were removed 10 days before and frozen earlier that morning.
No one ever transplanted frozen organs into large animals. There were a lot of things that didn't work out.
“I think there's about a 50% chance that it will work,” said Kolkout Wygun, a surgical professor and team leader, before the surgery. Dr. Uygun is on the Scientific Advisory Committee of Sylvatica Biotech Inc., a company that develops freezing methods for organ maintenance.
But the promise from the organs of freezing and storage is fantastic.
Kidneys may be severe and continuous shortages due to transplants – over 92,000 people are on the waiting list. One reason is that the 24-36 hour window is very short, so limit the number of recipients that are good matches.
How good is it to have a bank of stored frozen organs, as organ transplants can be like an elective surgery?
At least, it was a decades-long dream of a transplant surgeon.
However, the medical researchers' attempts to freeze organs were thwarted at every turn. In many cases, ice crystals formed organs and destroyed them. The material was also intended to stop the crystals from forming, the anti-freeze agent was toxic and killed cells. Or the frozen organs became very brittle and cracked.
After that, John Bischoff, a researcher in freeze biology at the University of Minnesota, had the problem of thawing organs, even though the freeze seemed to be on the way.
When they frozen the organs, scientists tried to confirm that the ice crystals that formed were very small. However, these crystals tend to grow when organs warm, cutting through delicate cells.
“As ice crystals grow, we have to overtake the ice crystals,” Dr. Bischoff said.
“The essential insight is that if all you do is warm it up at the edge, you can't go fast enough in the middle of the organ,” he said. “If heating only begins outside the frozen organ, the temperature difference from the edge to the center of the organ can lead to stress that breaks the organ, like an ice cubes that crack when placed in a drink.”
He added, “You have to heat evenly from the inside.”
His colleague, Dr. Eric Finger, also a transplant surgeon at the University of Minnesota, and although he was not involved in the mass common experiments at the University of Minnesota, he said that while freezing must be done slowly to prevent ice damage, it must recover 10-100 times faster than the cooling process.
Investigators messed with the system and eventually learned to successfully freeze, thaw and transplant rat kidneys.
However, larger animals introduced new problems.
“For 40 years, reuse has been a problem,” Dr. Finger said. “But increasing the size of the organs makes cooling a problem.” Suddenly, anti-freeze using small mouse organs was no longer sufficient.
In Massachusetts General, researchers attempted a different approach. It started with Shannon Tessier, a postdoctoral researcher in Dr. Uygun's lab and currently on the Sylvatica Biotech Advisory Board and associate professor of surgery at Harvard Medical University who has a patent application related to the methods used in the March surgery. A few years ago, she was studying the Canadian wood frog.
When the weather gets cold, the frog's metabolism changes, which itself can freeze. All cellular processes stop. That heart stops. It is essentially dead.
The frogs are very fragile, so the lab workers are very kind. “If you're not careful, you can break your arm,” said McLean Taggart, a lab technician.
“Shannon went into the lab and said, 'Is it possible to translate this into human organs?” Taggart said.
It worked to learn how frogs enter a deep freeze. Just before hibernation, the frog begins to produce large amounts of glucose. Glucose accumulates within the cells, where it reduces the freezing point of water and prevents ice from forming.
However, frogs are amphibians. Does something like that work in warm-blooded mammals and their organs?
You can see that is the case. Mammalian Arctic squirrels become super cool in themselves when temperature drops using a similar method. The cells reach temperatures below the freezing point of water – they are chilled, but not enough for ice to form. Its metabolism is very slow and there is no need to eat it.
Like their previous researchers, the Mass General group began with rat liver and tried to mimic the process. They decided to use the recently deleted organs, but still living organs, using the same process as wood frogs. It is cooled enough to stop the metabolic process, but not enough to risk the formation of large ice crystals.
They started by injecting artificial glucose that is unable to metabolize. Sugar accumulates in cells, but because it is not available, the cells enter the form of suspended animation, causing their metabolic processes to pause.
At the same time, the investigator adds diluted thawing – propylene glycol, which replaces the remaining water in the cells. As a result, very little ice forms inside the cells, causing damage caused by organ freezing.
Their storage solutions are a mixture of Snomax with diluted propylene glycol and artificial sugars, the substances used to make artificial snow on ski slopes. Snomax creates small, uniform ice crystals. This helps to ensure that the ice that forms does not cause damage.
To thaw the organs, the group reverses the process, placing the liver in a warm solution containing propylene glycol and artificial glucose, slowly diluting until the chemicals are gone.
Researchers said it takes about five years of trial and error to get the process right.
The next step was to move to a larger mammal species. They try to freeze and thaw pig kidneys.
Their ultimate goal was ambitious – they want to create the banks of frozen pig kidneys that have been genetically modified for use in human patients.
Other transplant surgeons at Dr. Wygun's hospital are beginning to experiment with the kidneys of genetically modified pigs. They implanted them into multiple human patients, resulting in mixed results. On Friday, the longest-lasting patient of her kidneys to date (130 days) had to remove it because her body refused.
No one knew whether the method used by Dr. Uygun and his colleagues would be successful.
“The protocol was optimized for the liver,” Dr. Uigun said. “We didn't think it would work.”
But it did.
The team tested the method, freezing and thawing 30 pig kidneys to ensure that the organs remained healthy after the freezing process. They learned that the kidneys can be frozen for up to a month without obvious damage.
But if transplanted into a pig, does previously frozen kidney function work?
In a test in March, the kidneys remained frozen for 10 days and were to be transplanted into the ingested pigs.
At 3am, the team began to thaw the kidneys. This took me two hours.
At 9am, General Massa's transplant surgeons, Dr. Alban Longchamp and Dr. Kawadoji opened the pig's abdomen and prepared the animals for surgery.
At 10:30 they sewed their kidneys.
The whitish-gray organ turned pink as soon as it was blood flowed.
Finally, success: before they sewed the pig, the researchers saw the implanted kidneys produce pee.